What if
we found cancer early enough to make a difference
Finding cancer early can improve survival
CancerScreenDx™ is a multi-cancer early detection test that can detect over 85 types of cancer through a single blood draw. This test is unique in that it can detect many types of cancer that are not commonly screened by routine cancer biomarkers tests.
When a cancer signal is detected, the CancerScreenDx™ test predicts the origin of the cancer signal with high accuracy to help guide the next steps to diagnosis. Using the CancerScreenDx™ test alongside existing screening tools is expected to improve early cancer detection for patients at an elevated risk of cancer, such as those aged 50 or older. This will help to identify cancer at an earlier stage, which can lead to improved outcomes for patients and reduced costs of treatment.
By offering this test, a health system can position itself as a leader in cancer detection and provide patients with valuable insights that can help them be proactive about their health.
How the test works
All cells, cancer and non-cancer, shed DNA into the bloodstream. As cancer progresses, the proportion of ctDNA in the blood tends to increase, making it a potential biomarker for the detection and monitoring of cancer.
The test can detect mutations in the ctDNA that are specific to a particular type of cancer, and by analyzing the mutation patterns, it can predict where in the body the cancer is located. This information can be used to guide the next steps in the diagnostic process, such as selecting the appropriate imaging tests or biopsies.
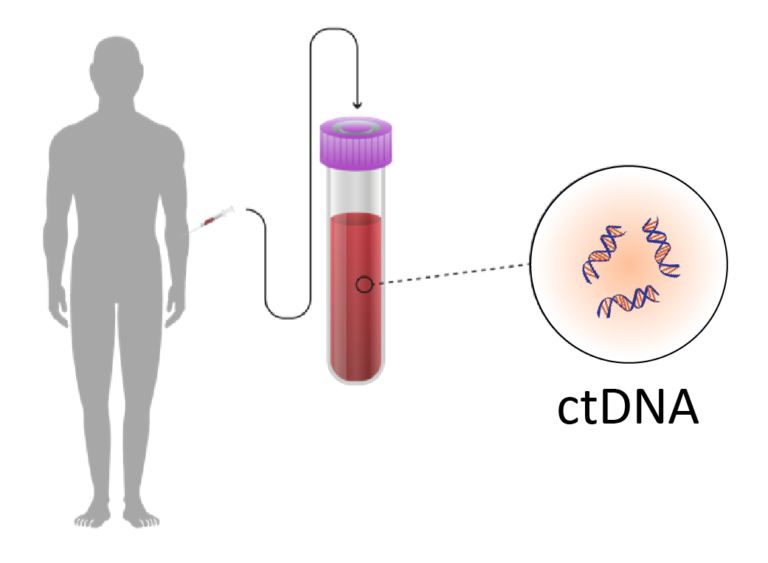
Understanding the results
The CancerScreenDx™ test detects potential indicators of cancer present in a blood sample. There are two possible results from the test:
Negative
This means that no cancer signal was found; however, not all cancers can be detected by the test. Be sure to continue with routine recommended cancer screening tests.
Positive
This means that there is a suspicion of cancer. The healthcare provider will discuss appropriate follow-up tests to confirm if cancer is present.
If the test result is positive, the report will also indicate the most likely source of the cancer signal. However, it is important to note that these results must be verified through additional diagnostic tests as per standard medical procedure. The results should also be considered in relation to the individual’s specific health risks. Even if a cancer signal is identified and the origin is not confirmed by diagnostic tests, there is still a heightened chance of cancer and further examination may be necessary. Our team at AGTC Genomics is here to assist you with any diagnostic follow-up and provide resources to support integration into your healthcare system.
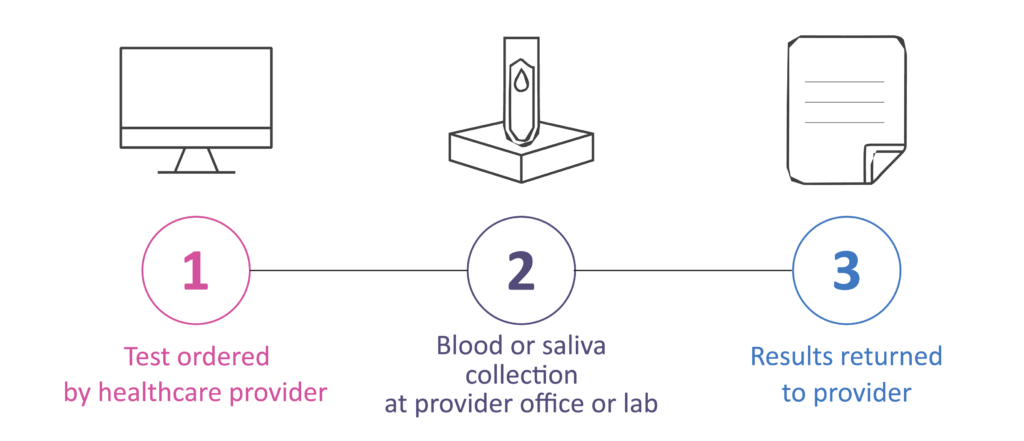
How to Order
Sample Preparation Instructions
All clinical materials should be collected with approved methods to avoid contamination and cross contamination. A sterile environment must be maintained when collecting samples. Our sample preparation requirements are detailed in the table below:
| Blood | ≥ 20mL blood collected in EDTA or cell free DNA collection tubes, no Heparin tubes allowed. |
| Plasma | Plasma samples should be collected and separated within 2 hours of collection, if possible, as longer times impact degradation and downstream sequencing success. Double spun plasma is also acceptable. |
| Frozen plasma | 5 - 10 mL plasma frozen at -80°C with no signs of haemolysis. |
Sample Transport
EDTA Blood can be sent via regular mail at room temperature. Plasma must be sent via refrigerated shipping. Frozen plasma must be sent via frozen shipping (with dry ice).
Shipping Instructions
Sample should be sent to:
AGTC Genomics Sdn Bhd (1428365-D)
J2-1, Pusat Perdagangan Bukit Jalil,
Jalan Persiaran Jalil 1,
Bukit Jalil,
57000 Kuala Lumpur,
Malaysia
References
1. Screening includes methods with USPSTF A or B rating. SEER*Stat Database: Incidence — SEER 18 Regs Research Data, Nov 2017.
2. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence — SEER 18 Regs Research Data, Nov 2018 Sub. Includes persons aged 50 – 79 diagnosed 2006 – 2015.
3. Banegas MP, Yabroff KR, O’Keeffe-Rosetti MC, Ritzwoller DP, Fishman PA, Salloum RG, Lafata JE, Hornbrook MC: Medical Care Costs Associated With Cancer in Integrated Delivery Systems. J Natl Compr Canc Netw 2018, 16(4):402-410.
4. Data on file from SEER 18 Regs Research Data, Nov 2017 Submission. Estimated deaths per year in 2020 from American Cancer Society Cancer Facts and Figures 2020.
5. Screening includes methods recommended by the United States Preventive Services Task Force (USPSTF) A, B, and C ratings.
6. Bronkhorst AJ, Ungerer V, Holdenrieder S: The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif 2019, 17:100087.
7. Oxnard GR, Klein EA, Seiden M, Hubbell E, Venn O, Jamshidi A, Zhang N, Beausang JF, Gross S, Kurtzman KN: Simultaneous multi-cancer detection and tissue of origin (TOO) localization using targeted bisulfite sequencing of plasma cell-free DNA (cfDNA). Annals of Oncology 2019, 30:v912.

