Enhanced Survival and Quality of Life
Hematopoietic cell transplantation is the predominant curative treatment for many malignant and non-malignant hematological diseases. Better pre-transplantation matching methods, treatment and follow-up has led to increased patient survival. Better post-transplant follow-up further improves the quality of life for these patients, as well as reduces health care costs. Early detection of graft rejection and disease relapse following allogeneic hematopoietic cell transplantation (HCT) improves patient outcomes by allowing treatment to be initiated as quickly as possible after the onset of relapse.
HLA-Typing for Donor Recipient Matching
Full HLA genes and additional transplant associated genes matching for pre-transplant testing.
Description : Pre-Transplantation High-Resolution HLA-Typing for Donor Recipient Matching
No. of genes : Maximum coverage of 17 loci including all exons for all genes
Sample Types : Blood
Input Quantity : ≥200 ng DNA
Post-Transplant Surveillance for Bone Marrow Transplant Patient
Engraftment monitoring (chimerism) testing evaluates the success of a hematopoietic stem cell transplant by measuring the relative ratio of the recipient and the donor cell population in the recipient’s post-transplant specimen. Engraftment monitoring may assist in detecting disease relapse or graft failure.
Description : Post-Transplantation-NGS-based chimerism monitoring for bone marrow transplant patient
No. of genes : Targets 202 bi-allelic SNPs across 22 autosomes
Sample Types : Blood
Input Quantity : ≥200 ng DNA

Post-Transplant Surveillance for Solid Organ Rejection Monitoring
Quantification of donor-derived cell-free DNA in transplant recipients. Measuring the level of donor-derived cell-free DNA may assist in improving transplant surveillance. Cell-Free DNA (cfDNA) is fragmented DNA in the bloodstream that originates from cells undergoing cell injury and death. When graft injury occurs, donor-derived cell-free DNA (dd-cfDNA) increases in the blood. cfDNA measures donor-derived cell-free DNA (dd-cfDNA) utilizing low DNA input, next generation sequencing technology, and streamlined analysis to assist in improved transplant surveillance.
Description : Donor-Derived Cell-Free DNA Testing for Organ Transplant Monitoring
Sample Types : Blood
Input Quantity : ≥200 ng DNA
Clinically Validated Across Multiple Studies
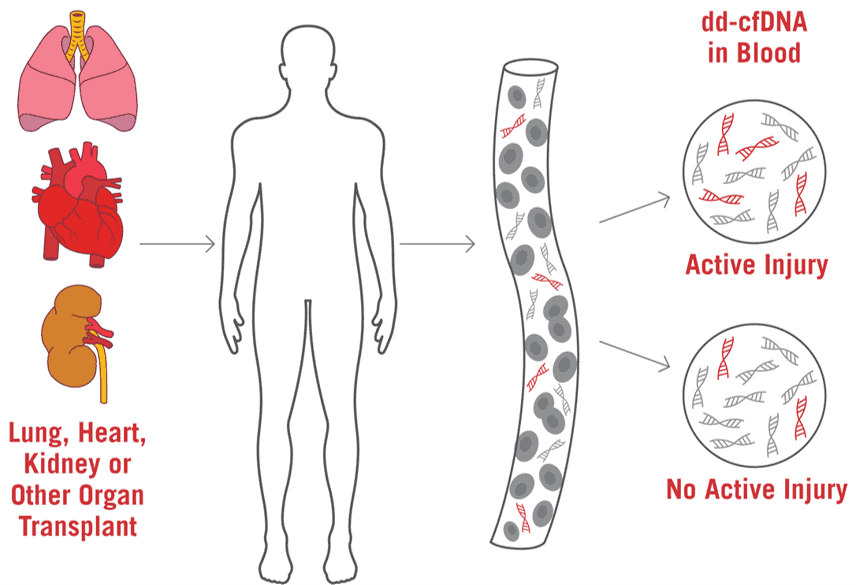
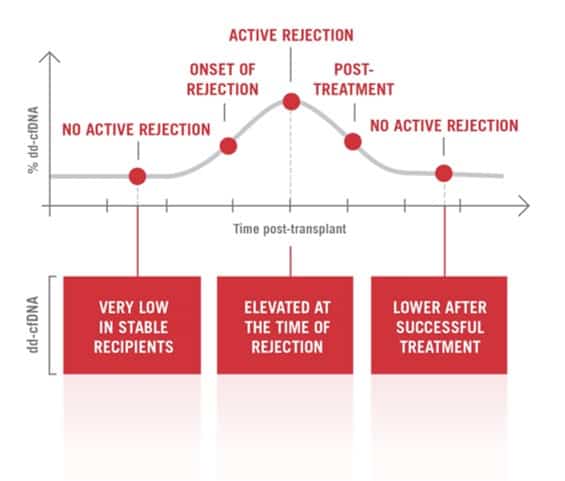
Cell-free (cfDNA) is fragmented DNA in the bloodstream that originates from cells undergoing cell injury and death. AlloSeq cfDNA™ measures cfDNA and uses single nucleotide polymorphisms (SNPs) to distinguish between donor and recipient. AlloSeq™ can quantify increasing levels of dd-cfDNA, serving as a leading indicator of graft injury.
Sample Preparation Instructions
All clinical materials should be collected with approved methods to avoid contamination and cross contamination. A sterile environment must be maintained when collecting samples. Our sample preparation requirements are detailed in the table below:
| EDTA Blood | ≥ 1 mL |
| Ready to use DNA | ≥ 1 µg For DNA samples, we recommend extraction using Qiagen kits, elution in water in 20 µL volume (min), plus evaluation of 260/280 Ratio (1.6 - 2.1) and fluorescent dye quantification of concentration (> 10 ng/µL) before sending. |
Sample Transport
EDTA Blood, buccal swab, saliva and DNA samples can be sent via regular mail at room temperature. Liquid blood is stable for up to 4 days during transport.
Shipping Instructions
Sample should be sent to:
AGTC Genomics Sdn Bhd (1428365-D)
J2-1, Pusat Perdagangan Bukit Jalil,
Jalan Persiaran Jalil 1,
Bukit Jalil,
57000 Kuala Lumpur,
Malaysia

