Unraveling Rare Disease Mysteries
The Value of Genomics in Diagnosing Rare Diseases
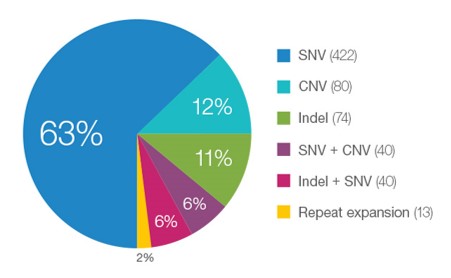
Many Different Variant Types are Pathogenic for Rare Disease Cases
Every Rare Disease Deserves Recognition
Join the shared quest to bring an end to the diagnostic journey by delving into the realm of rare disease genomics.
The Genome as the Foundation for Rare Disease Diagnosis
Discover the importance of whole-genome sequencing as the underlying support for all molecular genomics assays.
Advantages of Rare Disease Genomics
- TAILORED DISEASE MANAGEMENT: Comprehending the mechanisms of rare diseases enables physicians to direct patients to the right specialists, select personalized treatments, and provide condition-specific follow-up.
- REDUCED EXPENSES: By circumventing extended diagnostic journeys, genomic diagnoses for rare diseases can prevent expensive tests and procedures while minimizing unnecessary referrals.
- INFORMED FAMILY PLANNING: Determining the inheritance pattern of a rare disease offers insight into recurrence risks for patients and their families, facilitating knowledgeable family planning decisions.
- PSYCHOSOCIAL BENEFITS: Beyond reducing stress linked to diagnostic odysseys, obtaining a molecular diagnosis connects affected families with rare disease support groups, fostering a sense of community.
- WIDER SOCIETAL IMPACT: Delving into the genomics of rare diseases can help identify novel drug targets and enhance the effectiveness of care.
Rare Disease Patient Stories
Ending Carson & Chase’s 6-Year Diagnostic Odyssey
Initially, Carson received a cerebral palsy diagnosis, but his brother Chase’s condition revealed this to be incorrect. After another four years, whole-genome sequencing successfully identified the true diagnosis for both Carson and Chase: mitochondrial enoyl CoA reductase protein-associated neurodegeneration (MEPAN).
Donovan’s Journey to Diagnosis
Donovan’s range of symptoms pointed to over 20 possible conditions and nearly a dozen medical specialties. After a 6-year search, whole-genome sequencing detected a variant in the SKI gene, ultimately leading to a diagnosis of Shprintzen-Goldberg syndrome.
Sample Preparation Instructions
All clinical materials should be collected with approved methods to avoid contamination and cross contamination. A sterile environment must be maintained when collecting samples. Our sample preparation requirements are detailed in the table below:
| EDTA Blood | ≥ 1 mL |
| Buccal swab | 1 Oragene™ OCR-100 buccal swab collection set |
| Saliva | 1 Oragene™ OG-610 saliva collection set |
| Ready to use DNA | ≥ 1 µg For DNA samples, we recommend extraction using Qiagen kits, elution in water in 20 µL volume (min), plus evaluation of 260/280 Ratio (1.6 - 2.1) and fluorescent dye quantification of concentration (> 10 ng/µL) before sending. |
Sample Transport
EDTA Blood, buccal swab, saliva and DNA samples can be sent via regular mail at room temperature. Liquid blood is stable for up to 4 days during transport.
Shipping Instructions
Sample should be sent to:
AGTC Genomics Sdn Bhd (1428365-D)
J2-1, Pusat Perdagangan Bukit Jalil,
Jalan Persiaran Jalil 1,
Bukit Jalil,
57000 Kuala Lumpur,
Malaysia
References
1. Nguengang Wakap, S., Lambert, D.M., Olry, A. et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28:165–173. https://doi.org/10.1038/s41431-019-0508-0
2. Ferreira CR. The burden of rare diseases. Am J Med Genet A. 2019;179(6):885-892. doi:10.1002/ajmg.a.61124
3. Walker CE, Mahede T, Davis G, et al. The collective impact of rare diseases in Western Australia: an estimate using a population-based cohort. Genet Med. 2017;19(5):546-552. doi:10.1038/gim.2016.143
4. Global Commission to End the Diagnostic Odyssey for Children with a Rare Disease. 2019.
5. Rare Disease Impact Report: Insights from patients and the medical community. globalgenes.org/wp-content/uploads/2013/04/ShireReport-1.pdf.
6. Bick D, Jones M, Taylor SL, Taft RJ, Belmont J. Case for genome sequencing in infants and children with rare, undiagnosed or genetic diseases. J Med Genet. 2019;56(12):783-791. doi:10.1136/jmedgenet-2019-106111.
7. Clark MM, Stark Z, Farnaes L, et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom Med. 2018;3:16. https://doi.org/10.1038/s41525-018-0053-8
8. Vissers LE, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nat Rev Genet. 2016;17(1):9-18. doi:10.1038/nrg3999
9. ACMG Board of Directors. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2015;17(6):505-507. doi:10.1038/gim.2015.41

